Temperature Conversion Tool
While we try to provide 100% accuracy in our online tools, we cannot guarantee it – Please use this tool for a quick reference only.
Measuring Temperature
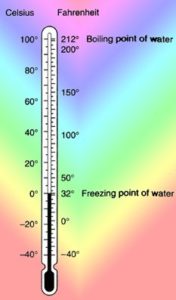
Several tools have been devised to correctly find temperature level. With the advent of a temperature scale, the accurate measurement of temperature got started. This scale revolutionized the process of temperature measurement into meaningful numbers. It was in the initial phase of the eighteenth century, Gabriel Fahrenheit (1686-1736) invented the Fahrenheit scale. He kept the water freezing point at 32 degrees and the boiling point at 212 degrees. The purpose was to form the anchors with two different points for his scale. In the subsequent years of that century during 1743, it was Anders Celsius (1701-1744), who created the Celsius scale. Based on the principle of two points discovered by Fahrenheit, Celsius considered the water freezing temperature to be 0 degree and the boiling temperature to be 100 degrees. The Celsius scale is referred to as a Universal System Unit. It has been prevalent since then in all parts of the globe to accurately measure temperature. There is no denying the fact that the severity of the coldness varies accordingly, however, the Kelvin scale is formulated to go to zero at this minimum temperature. The relevance between the different temperature scales are:
oK = 273.15 + oC oC = (5/9)*(oF-32) oF = (9/5)*oC+32
| oF | oC | oK | |
| Water boils | 212 | 100 | 373 |
| Room Temperature | 72 | 23 | 296 |
| Water Freezes | 32 | 0 | 273 |
| Absolute Zero | -460 | -273 | 0 |
When the temperature reaches Absolute Zero, no object is found to be in motion and emits heat. Absolute zero means all atomic and molecular motion freezes and is at the least possible temperature. Absolute Zero occurs at 0 degrees Kelvin or -273.15 degrees Celsius or at -460 degrees Fahrenheit. All objects release thermal energy or heat till they are in a state at a temperature of absolute zero.
If we wish to comprehend the role of temperature on the molecular level, we must be aware of the fact that temperature is the average vivacity of the molecules that comprise of a substance. The atoms and molecules in a substance hardly remain in motion at an equal speed. This clearly indicates that there is a magnitude of energy (the energy of motion) among the molecules. In a gas, for instance, having molecules that remain in motion at a variety of speeds – some are quick to move and some still remain slow while in motion. Sometimes these molecules collide with each other. When this occurs, the molecule in motion at a great speed transfers some of its energy to the slower molecule, impelling the slower molecule to improve its speed and the faster molecule to slow down. The more energy is emitted to the system, the higher will be the speed of molecules carrying average, resulting in the emission of more thermal energy or heat. As a result, the rise in temperatures means a substance is featured with higher average molecular motion. We do not assume or observe a group of varied temperatures for each molecule which has a different speed. What we gauge as the temperature is usually associated with the average speed of the molecules in a system.
Click here To know more, peruse a java applet which describes how molecules move at different temperatures.
There is a revelation that the molecules move slowly in an object which is colder and they move fast when the object is hotter. And when both objects get collided, thermal equilibrium is reached because the molecules of both objects move at same average speed, thus indicating that the speed of motion remains same.
For more information please call us: 1-409-861-0788 or toll-free at 1-866-861-0788.

